The Pharmaceutical Supervision and Administration Bureau (ISAF) said in a statement yesterday that its inspectors detected a trading company in the Barrier Gate checkpoint area that was supplying Primovir, an oral generic medicine used for treating COVID-19, adding that the medicine, which was manufactured in India, was imported without the bureau’s permission.
The statement underlined that the bureau will refer the suspected illegal import case to the Macau Customs Service for breaching the External Trade Law.
According to the statement, the bureau has been carrying out inspections of local pharmacies, during which eight trading companies in the Barrier Gate checkpoint area were found to be suspected of illegally importing and supplying drugs.
The statement underlined that only two oral Western medicines – Paxlovid tablets and Lagevrio capsules – produced by Pfizer and Merck, have been approved for import into Macau. The statement stressed that both oral drugs have a range of contraindications and precautions. Therefore, the statement noted, they are currently only available by prescription from the Health Bureau (SSM).
As the safety, quality and effectiveness of drugs imported from unknown or unauthorised sources cannot be guaranteed, the bureau urged residents not to buy or take them.
Residents may contact the bureau on 8598 3522 during office hours or 8598 3504 for 24-hour telephone recording or via email at dli@isaf.gov.mo if they find any businesses suspected of illegally supplying drugs, the statement said.
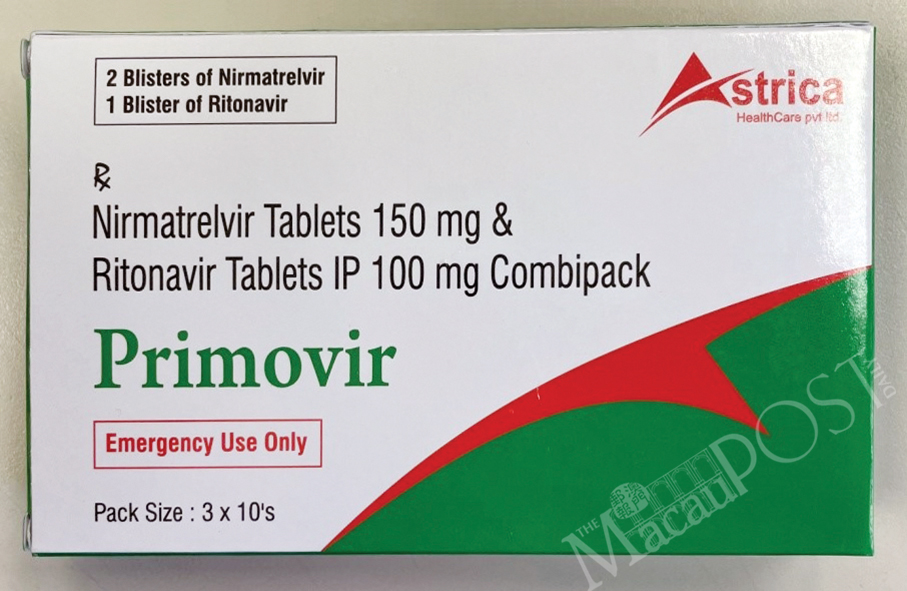
This undated handout photo provided by the Pharmaceutical Supervision and Administration Bureau (ISAF) yesterday shows the oral generic COVID-19 medicine Primovir illegally imported from India.







