An adapalene-benzoyl peroxide topical gel under the brand name Epiduo Forte, produced by Canada’s Galderma Production Incorporated, has been withdrawn from the local drug market as a precaution, the Pharmaceutical Supervision and Administration Bureau (ISAF) said in a statement yesterday.
The statement pointed out that during a stability test, the manufacturer found that the topical gel with the batch number 359448 contained a “slightly higher” amount of the active ingredient benzoyl peroxide than indicated on its label.
According to the statement, the problematic product, which is a topical medicine used to treat acne, had been supplied to three local medical centres. The statement underlined that the product has been recalled by a local pharmaceutical importer from the three centres.
Enquiries can be made by calling 8598 3533 during office hours.
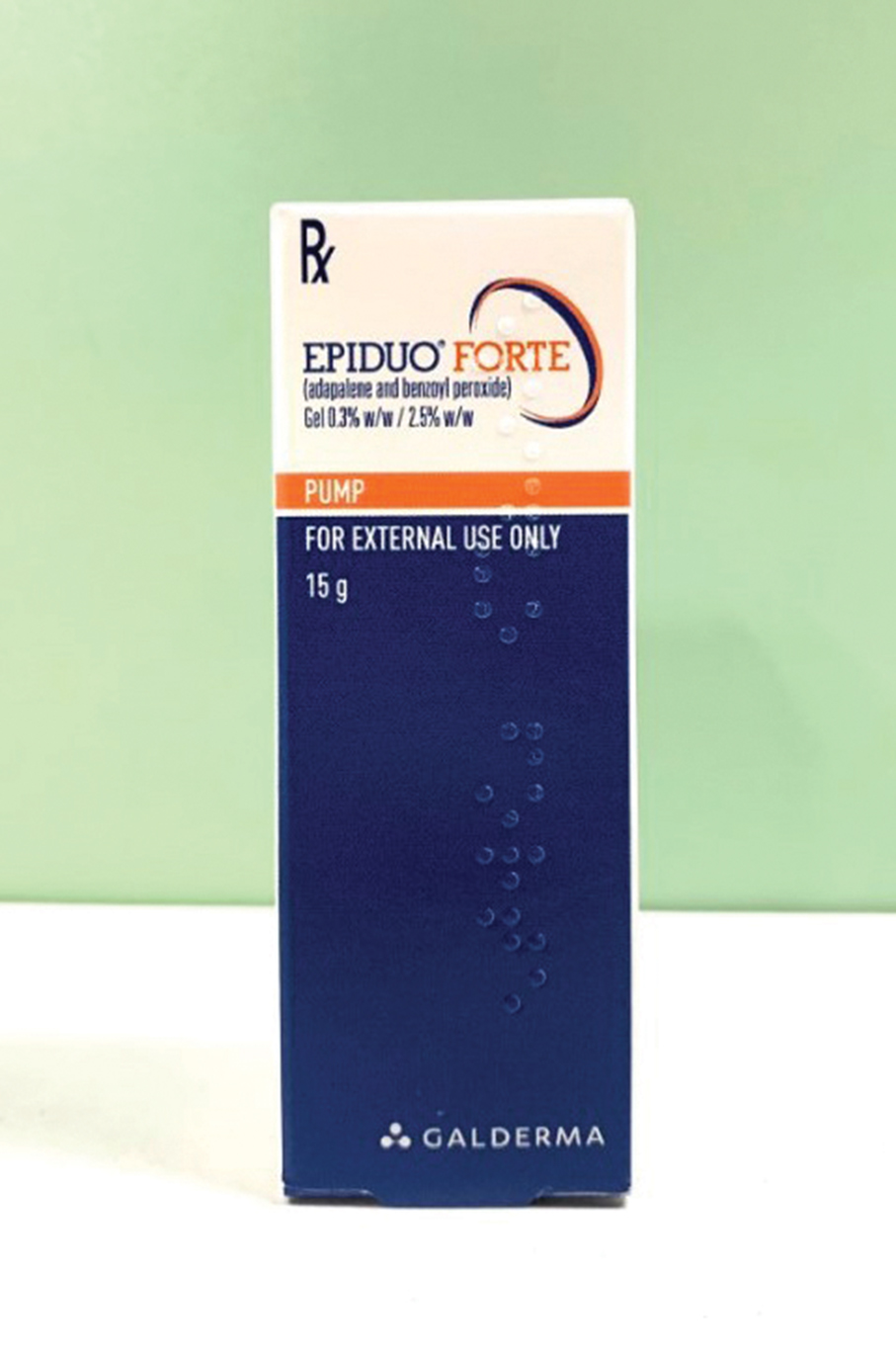
This undated handout photo provided by the Pharmaceutical Supervision and Administration Bureau (ISAF) yesterday shows a box of Epiduo Forte adapalene-benzoyl peroxide gel from Canada that has been withdrawn from the local market.









