The Pharmaceutical Supervision and Administration Bureau (ISAF) said in a statement last night that it was recalling an anti-epilepsy drug – Sabril Tab 500mg 100’s – manufactured by Patheon France as one of the batches with lot number 2991A was found to contain a trace amount of the impurity tiapride due to residues in the collinear production equipment.
The statement noted that the amount of tiapride content was found to be lower than the acceptable daily exposure.
As a precautionary measure, according to the statement, the licensed wholesaler of the medicine in Hong Kong decided to voluntarily recall the said batch of the affected medicine.
The statement pointed out that the medicine containing vigabatrin is a prescription drug used to treat epilepsy.
According to the information provided by the importer, the statement said, the affected batch of the drug had been supplied to the public Conde de São Januário Hospital Centre and local pharmacies.
The statement underlined that the bureau has followed up on the supply of the medicine and has notified the public hospital and relevant pharmacies to recall the drug. The statement also stressed that the medicine supplied to the public hospital has not been dispensed yet, therefore, no patient has been given the drug which is currently sealed.
In the statement, the bureau urged the pharmacies to immediately cease distribution and supply of the affected batch of the drug.
Residents who obtained the problematic drug at a local pharmacy are urged to consult the attending physicians or respective pharmacists to handle the product appropriately, according to the statement.
Enquiries can be made by calling 8598 3533 during office hours.
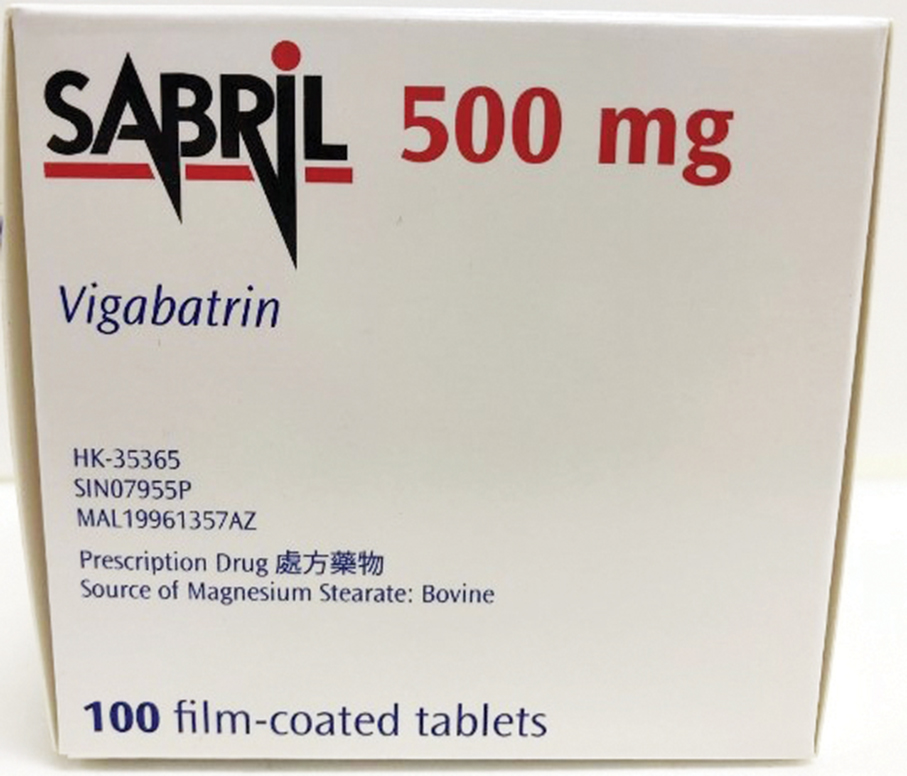
This undated handout photo provided by Pharmaceutical Supervision and Administration Bureau (ISAF) last night shows a box of the French-made anti-epilepsy drug, Sabril Tab 500mg 100’s.








