The Health Bureau (SSM) said in a statement yesterday that it is recalling “Witt Cestasin Capsules” manufactured by Vacopharm Pharmaceutical Joint-Stock Company in Vietnam because the product’s microbial limits exceeded the established pharmacological criteria.
According to the statement, the problematic remedy’s batch number is 1090319. The product is used to treat rhinitis. The statement pointed out that the bureau had instructed the importers of the product to recall it.
The statement said that according to the import records, the remedy is sold in community pharmacies. The bureau urged residents who have bought the product to contact a pharmacist. It did not ask them to stop using the product.
For more information, contact the Health Bureau on 85983453.
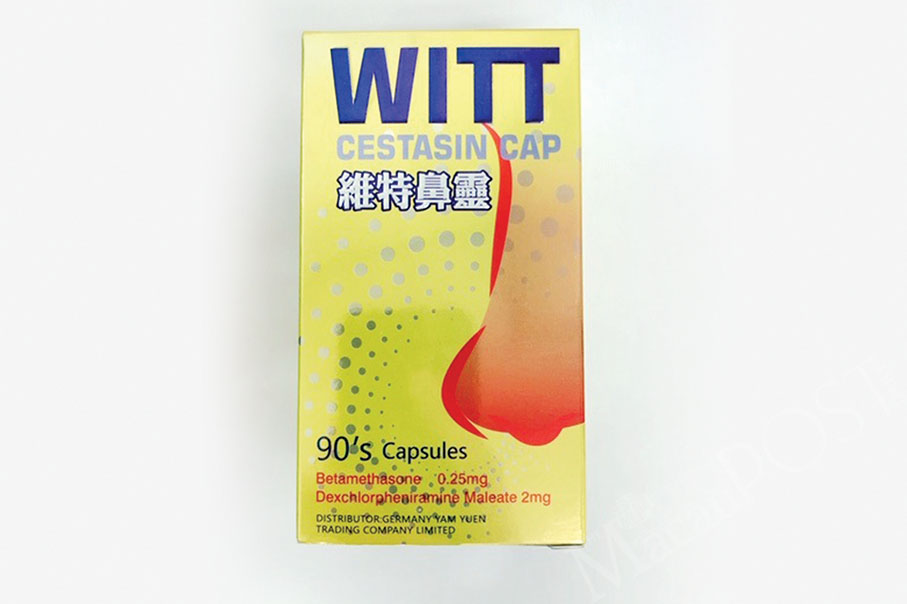
This undated handout photo provided by the Health Bureau yesterday shows the packaging of Witt Cestasin Capsules, which is being recalled.






