The Pharmaceutical Supervision and Administration Bureau (ISAF) warned the public in a statement on Friday about two topical ointments that have been found to contain the unapproved pharmaceutical ingredients Mometasone furoate and Miconazole, a steroid medication typically used to treat inflammation and fungal infections,
According to the statement, the bureau has recently been warned by the Hong Kong Department of Health that two topical ointments, “Eczevandia Cream” and “Eczevandia Baby Cream”, contain Mometasone furoate and Miconazole, which must be used under medical supervision.
The bureau emphasised that these products have not been approved for import or sale in the local market, the statement said, adding that in order to protect public health, the bureau urged residents to immediately stop using these products to avoid potential health risks.
Mometasone furoate can cause a range of side effects, including high blood pressure, elevated blood sugar, skin atrophy, adrenal insufficiency, and osteoporosis, the statement said, while Miconazole may lead to local skin irritation and allergic reactions, according to the statement.
The bureau vowed to closely monitor the circulation of the two ointments in Macau. Members of the public who have purchased these products should stop using them immediately and take them to the bureau for proper disposal.
For any enquiries, members of the public can call 8598 3233 during office hours.
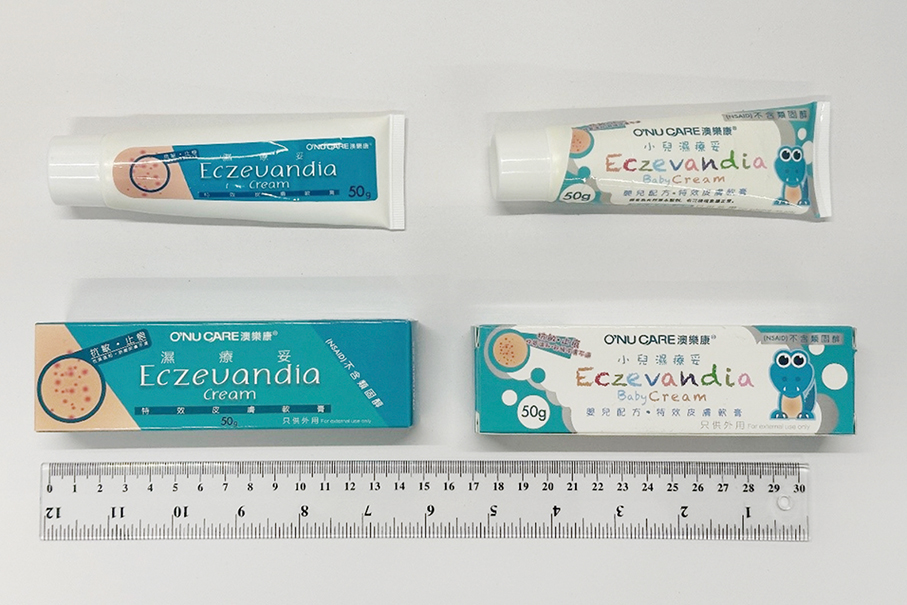
This undated handout photo provided by the Pharmaceutical Supervision and Administration Bureau (ISAF) on Friday shows two topical ointment products that were found to contain unapproved pharmaceutical ingredients Mometasone furoate and Miconazole.