The Pharmaceutical Supervision and Administration Bureau (ISAF) said in a statement on Friday that Batch No. RN3T of antiviral drug “ZOVIRAX IV INFUSION INJ 250MG”, manufactured by Italy-based GlaxoSmithKline Limited has been recalled from the public Conde de São Januário Hospital Centre after being found to contain glass particles in one of its bottles.
The product is a prescription medication used to treat simple herpes and varicella shingles infections, the statement noted.
According to the information provided by the importer, the affected batch of the drug was only supplied to the public hospital centre, and the bureau has instructed the importer to follow up on the recall.
According to the public hospital centre, it has immediately stopped using the affected batch of the drug, which is only for in-hospital use, and it has not received any reports of patients feeling unwell after using the drug, and will keep a close watch on the situation.
Residents who are in doubt can seek advice from their attending doctors or call the bureau on 8598 3233 during office hours.
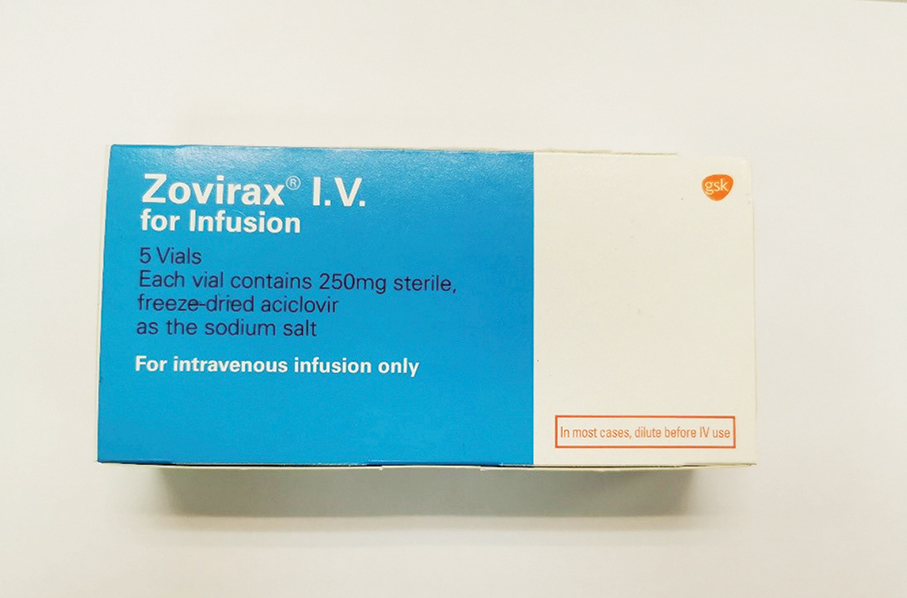
This undated handout photo provided by the Pharmaceutical Supervision and Administration Bureau (ISAF) on Friday shows ‘ZOVIRAX IV INFUSION INJ 250MG’, produced by Italy-based GlaxoSmithKline Limited.








