The Health Bureau said in a statement last week that it has instructed local wholesalers, pharmacies and clinics to recall six products containing valsartan from the market because an impurity has been detected.
The six affected products are: “Valtensin Tablets 80mg”, “Valtensin Tablets 160mg”, “Valtensin HCT Tablets 80/12.5mg”, “Valtensin HCT Tablets 160/12.5mg”, “Valsartan + Hidroclorotiazida Generis 80mg + 12.5mg”, and “Valsartan + Hidroclorotiazida Generis 160mg + 12.5mg”, according to the statement issued on Friday.
According to Wikipeida, valsartan (trade name Diovan) is mainly used for treatment of high blood pressure, congestive heart failure, and to increase the chances of living longer after a heart attack.
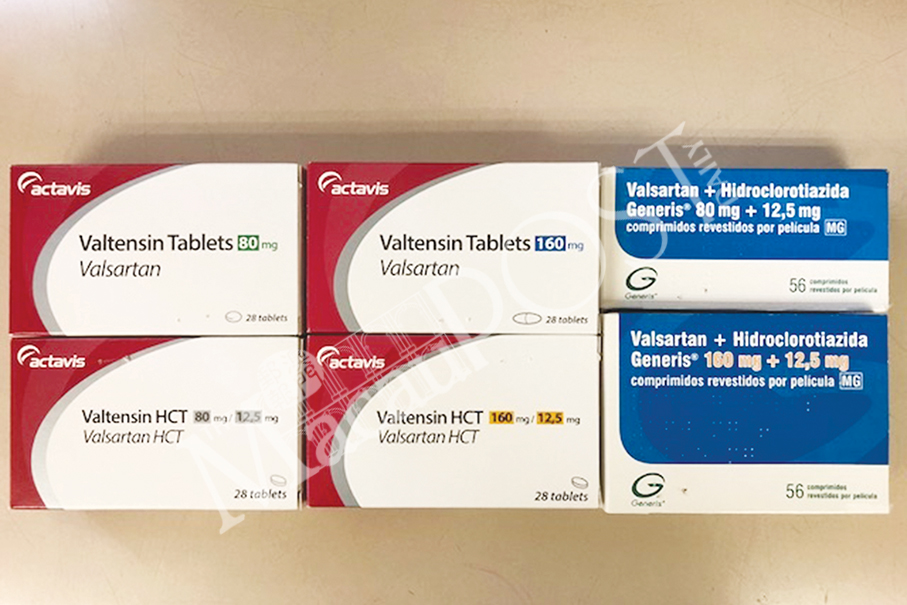
This undated handout photo provided by the Health Bureau (SSM) last Friday shows the six products containing valsartan, which are being recalled due to an impurity which poses a potential cancer risk.







