The Health Bureau (SSM) said in a statement yesterday that it is recalling three kinds of hypertension medicines, all manufactured by the same French pharmaceutical company, Sanofi, for having excessive amounts of impurities.
The statement said that the affected products are as follows:
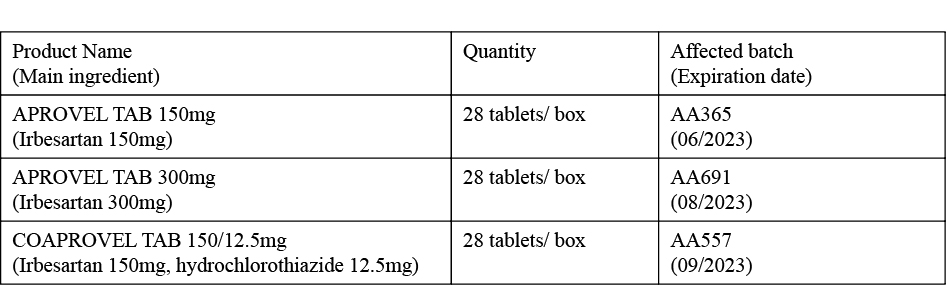
According to the statement, the affected batch of medicine had been sold to Macau’s community pharmacies, private clinics and private hospitals. The statement added Sanofi has an agreement with the bureau to supply APROVEL TAB 150mg and APROVEL TAB 300mg.
The statement said in order to protect public health, the Health Bureau (SSM) urges all pharmacies, hospitals, clinics and importers to recall the affected medicines. The statement also said that residents that have been prescribed the affected medicine can consult a pharmacist about the way to properly handle the products. However, the statement underlined that residents who have had the medicine prescribed by doctors should contact the clinic or hospital to make new arrangements, urging them not to abruptly pause taking the medicine. For any enquiries, one can contact the bureau on 8598 3441.


These undated handout photos provided by the Health Bureau (SSM) yesterday show the affected high blood pressure medicines.








