The Pharmaceutical Supervision and Administration Bureau (ISAF) announced on Friday that Batch Nos. U019788, U027875, U037539, W001030 and W026080 of the Belgium steroid medicine “Diprospan Injection 1ml”, which was manufactured by Schering-Plough Labo N.V., have been withdrawn from the local market.
An ISAF statement on Friday noted that the bureau was notified by the local importer of the drug that as the manufacturer found stainless steel particles in the product, it voluntarily recalled all problematic batches of the medicine from the market as a precautionary measure.
According to the statement, the drug containing betamethasone dipropionate and betamethasone sodium phosphate is a prescription drug used to treat inflammation.
The statement said that the batches of drug were supplied to a number of local medical institutions, underlining that the importer has notified them to recall the drugs and that the medical institutions should immediately stop using the batches.
The statement stressed that the bureau will continue to pay close attention to the issue.
Enquiries can be made by calling 8598 3533 during office hours.
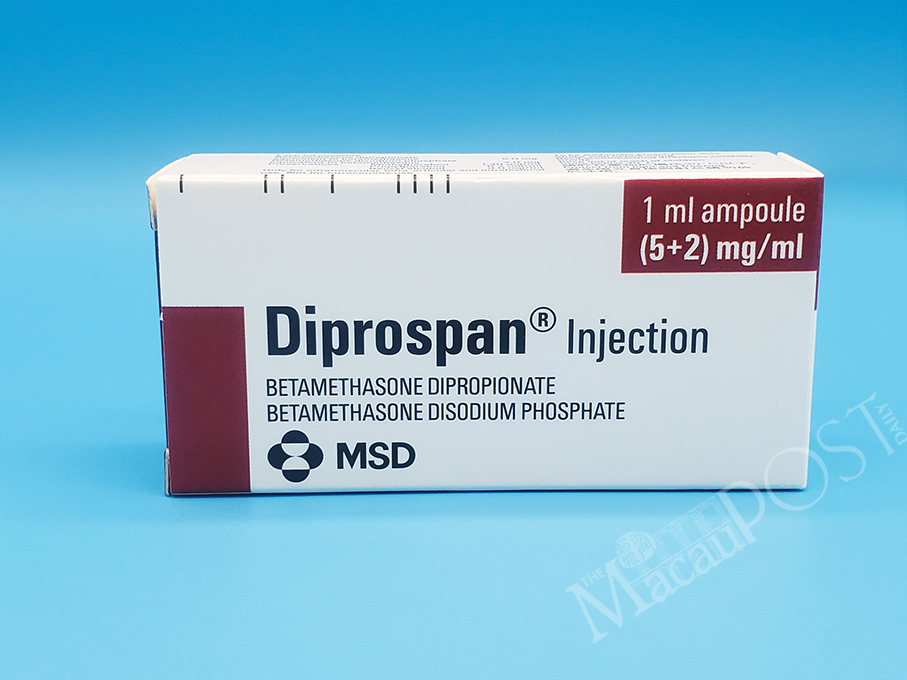
This undated handout photo provided by the Pharmaceutical Supervision and Administration Bureau (ISAF) on Friday shows a box of the steroid medicine “Diprospan Injection 1ml” manufactured in Belgium.









